About the ROADMAP project
BACKGROUND
There are anywhere from 7,000 to 10,0001 rare diseases affecting approximately 30,000,0002 individuals in the U.S.A. Despite significant investments of resources over the last several decades, most of these rare diseases do not have a single FDA-approved therapy, some estimate that over 90% of rare diseases lack an effective treatment (Kaufmann et al., 2018; Orphanet J Rare Dis). This fact highlights the failure of traditional research approaches to overcome the unique challenges faced by rare disease research.
In the rare disease space, rare disease non profit organizations are often key players in supporting the patient community through outreach, advocacy, fundraising, financial support, treatment guidance, as well as supporting the researchers and physicians in advancing understanding and treatment options for the rare disease. Since the incentives for new drug development for rare diseases are limited, drug repurposing provides a promising way to identify effective treatments for rare diseases.
The basic idea of drug repurposing is that once a treatment has been FDA-approved for one condition, it can be re-approved, or repurposed, to treat other conditions. The same goes for drugs developed for one condition, but proven ineffective or unsafe and thus never approved for the intended condition (this is sometimes referred to as “drug rescue”).
The repurposing process, in most cases, is considered to be a faster and cheaper way of getting treatments to patients compared to developing new drugs. Because the basic research on the properties of the compound has already been completed, as well as preliminary safety clinical trials, drug repurposing is able to skip many steps and costs. Depending on the new indication and how different it is from the original in both the target disease, population, dosages and modes of administration, drug repurposing could start from Phase II or Phase III clinical trials, and thus it becomes a faster (only 1-3 years to implementation, comparing to the 13-15 years for new drug development) and cheaper ($300 million, compared to $2-3 billion for new drug development (Pushpakom et al., 2019) option to get treatments to patients.
Additionally, because the repurposed drug has already been proven safe and effective, it has a higher likelihood to succeed in future clinical trials, compared to new, untested compounds - 1/10 success rate vs the 1/10,000 for new drug development (Stone, 2020).
However, the process has not received enough interest from relevant stakeholders or funding organizations, and most prior drug repurposing efforts are a result of serendipitous discovery rather than a systematic, intentional pursuit (Pushpakom et al., 2019). This is because many challenges exist in the drug repurposing space, namely:
- lack of a consensus of the roles that various stakeholders play (patient advocacy organizations, researchers, physicians, government, pharmaceutical companies, etc.)
- lack of a “roadmap” of how rare disease organizations should go about pursuing drug repurposing,
- lack of support (data, finances, resources, guidelines, etc.) during the drug repurposing process.
ACKNOWLEDGEMENTS
We wish to the thank the following individuals and organizations for their contributions to this important work:
CDCN Team
Dr. David Fajgenbaum, Ania Korsunska, Mileva Repasky, Mary Zuccato, Sarah E. Bolden, Trae Boyd
and prior team members Johnson Khor and Dr. Ruth-Anne Langan Pai.
Participating rare disease nonprofit organizations
Acromegaly Community Inc, Adult Polyglucosan Body Disease Research Foundation, Aicardi-Goutieres Syndrome Americas Association, AKU Society of North America, Alport Syndrome Foundation, Amyloidosis Support Groups Inc., Angelman Syndrome Foundation, ASXL Rare Research Endowment Foundation, Autoinflammatory Alliance, Barth Syndrome Foundation, Ben’s Friends Patient Communities, Beyond Batten Disease Foundation, Bow Foundation, BPAN Warriors, CACNA1A Foundation, Campaign Urging Research for Eosinophilic Disease, Cardio Facio Cutaneous International, Castleman Disease Collaborative Network, CDG CARE, Champ Foundation, CHAMP1 Research Foundation, Chelsea’s Hope Lafora Children’s Research Fund, Child Neurology Foundation, Children’s Tumor Foundation, Cholangiocarcinoma Foundation, Chordoma Foundation, Choroideremia Research Foundation, Chronic Recurrent Multifocal Osteomyelitis Foundation, Circadian Sleep Disorders Network, CLOVES Syndrome Community, Coalition to Cure Calpain 3, Congenital Hyperinsulinism International, Costello Syndrome Family Network (CSFN), CSNK2A1 Foundation, Cure CMD, Cure GM1 Foundation, Cure HHT, Cure JM Foundation, Cure VCP Disease, Inc., cureCADASIL Association, CureSearch for Children’s Cancer, Cyclic Vomiting Syndrome Association, Daphne’s Lamp, DDX3X Foundation, DeSanto-Shinawi Syndrome, Dreamsickle Kids Foundation, DRESS Syndrome Foundation, Dup15q Alliance, E.WE Foundation, Emily’s Entourage, Epilepsy Alliance America, Facial Pain Association, Familial Chylomicronemia Syndrome (FCS) Foundation, Fanconi Anemia Research Fund, Foundation for CAMK2 Therapeutics, Foundation for Ichthyosis and Related Skin Types, Foundation for Sarcoidosis Research, FOXG1 Research Foundation, FPIES Foundation, GACI Global, Glut1 Deficiency Foundation, Hannah’s Hope Fund, Hermansky-Pudlak Syndrome Network, Hugs For Mito, Inc., Huntington’s Disease Society of America, Hypersomnia Foundation, Hypoparathyroidism Association, IDefine, Indian Organization for Rare Diseases, Infantile Neuroaxonal Dystrophy Cure Foundation, International Autoimmune Encephalitis Society, International Fibrodysplasia Ossificans Progressiva Association, International Pemphigus Pemphigoid Foundation, Jamal’s Helping Hands Inc., Jansen de Vries Syndrome Foundation, Jansen’s Foundation, Jordan’s Guardian Angels, Kabuki Syndrome Foundation, KIF1A.ORG, Koolen-de Vries Syndrome Foundation, LAM Foundation, Life Raft Group, Liv4TheCure, Lymphangiomatosis & Gorham’s Disease Alliance, Malan Syndrome Foundation, Mast Cell Hope, MEPAN Foundation, Mission: Cure, Mississippi Metabolics Foundation, Muscular Dystrophy Association, Myasthenia Gravis Foundation of America, Myositis Support and Understanding Association, National Foundation for Ectodermal Dysplasias, National Neutropenia Network, National Organization for Disorders of the Corpus Callosum, National PKU News, NEC Society, NephCure Kidney International, Neurodegeneration with Brain Iron Accumulation Disorders Association, Neurofibromatosis Northeast, Ocular Melanoma Foundation, Oligo Nation, Organic Acidemia Association, Pachyonychia Congenita Project, PCDH19 Alliance, Pityriasis Rubra Pilaris (PRP) Alliance, Platelet Disorder Support Association, Primary Ciliary Dyskinesia (PCD) Foundation, Project ALS, Progressive Familial Intrahepatic Cholestasis (PFIC) Network, PSC Partners Seeking a Cure, PTEN Hamartoma Tumor Syndrome Foundation, RASopathies Network, Raymond A. Wood Foundation, Recurrent Respiratory Papillomatosis Foundation, Remember The Girls, RUNX1 Research Program, RYR-1 Foundation, Sara’s Cure AKA Clear Cell Sarcoma Foundation, SATB2 Gene Foundation, Shwachman-Diamond Syndrome Foundation, Siegel Rare Neuroimmune Association, SLC6A1 Connect, Smith-Kingsmore Syndrome Foundation, Smith-Lemli-Opitz /RSH Foundation, Snyder-Robinson Foundation, Sophie’s Hope Foundation, Spastic Paraplegia Foundation, Spinal CSF Leak Foundation, Stiff Person Syndrome Research Foundation, STXBP1 Foundation, Sumaira Foundation, Superficial Siderosis Research Alliance, Syngap1 Foundation, T.E.A.M. 4 Travis (Together Ending Asplenia Mortality), Tatton Brown Rahman Syndrome Community, TBCK Foundation, Team Telomere, United States Thrombotic Microangiopathy Alliance, Usher 1F Collaborative, Usher Syndrome Coalition, Vici Syndrome Foundation, Inc, Pulmonary Alveolar Proteinosis (PAP) Foundation, TESS Research Foundation, and all participating patients, loved ones, researchers and physicians.
Volunteers
Meg Zuccato, Anna Nguyen, Derek Ansel, Martin Lukac, Annalise Jear, Mitav Nayak, Panchatapa Baul, Samatha Hood, Sabina Grigorian, Jacob Lowy, Justin Wong, Sydney Grisham, Yuan (Abby) Feng, Leanna Chen, Megan Shieh, Bryan Aguilar, Marcy Spiker, Rose Weathers, Katherine Fang, Veikko Toikka, Robert Parillo, Lindsay McBride, Sara Barrett, Robert Parillo, Dallas Ryan, Veikko Toikka, Benita Balogun, Penny Deremer, Miti Patel, Emma Roemer, Neda Pazuki, Erikka Chowdhury, Stephanie Hage, Matt Scott, Geetha Turlapati, Jessica Xiang, Jada Watkins, Jazmin Loughlin, Justin Crawmer, Ferzana Niazi, Anaheit Arathoon, Michael Zhang, Vee Suresh, Mahima Sangtani, Sara Cronin, Rita Aberbach, Kayleigh Nicole Murray, Diane Baynes, Andrew Zhu, Anastasia Kakurina, Susanna Hunanyan, Jade Bondy, Simarsukh Dhillon, Angela Perry, Carolyn Canterbury and Owen Yu.
Partners & Collaborators
Chan Zuckerberg Initiative, Medidata Solutions
Other
Beacon, Rare Revolution Magazine, CDRD, PTEN Research
METHODS
The Repurposing Of All Drugs, Mapping All Paths (ROADMAP) project aims to answer some fundamental questions about the experience of drug repurposing for rare disease nonprofit organizations and their relevant stakeholders and to design a solution to some of the challenges rare disease organizations are facing through the creation of this interactive “ROADMAP” tool. This project is supported by a grant from the Chan Zuckerberg Initiative (CZI).
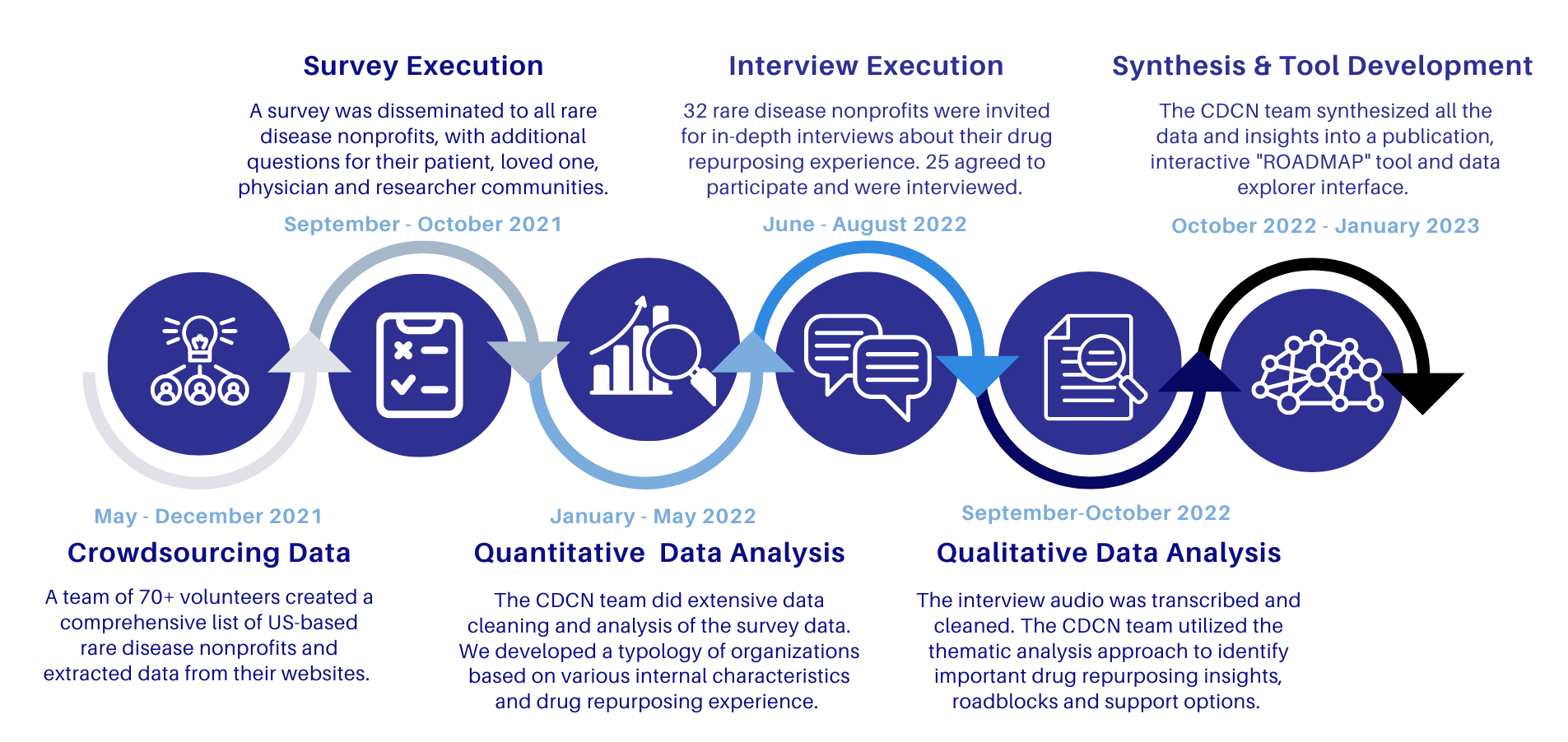
The CDCN team established a six-phase project plan for the project:
- Crowdsourcing Data
- Survey Data Analysis
- Interview Execution
- Interview Data Analysis
- Synthesis & Tool Development
This project was submitted for review by Advarra IRB under protocol number Pro00055201 and approved on September 28, 2021. On May 14, 2022 we submitted an update to the protocol, which included our interview protocol and updated project timeline, which was approved May 25, 2022.
Crowdsourcing Data
All Rare Disease Nonprofit Organizations in the US
The first dataset we needed was a comprehensive list of all rare disease nonprofit organizations in the US and an understanding of how many of them support research in general and drug repurposing in particular. We quickly discovered this dataset did not exist. In order to gather this data, we put out a call to assemble a team of volunteers through various social networks. We onboarded a small team of volunteers and commenced the first step of our crowdsourced data collection, which consisted of combining several existing lists of organizations (NORD members, Global Genes members, etc.) and then performing additional searches to try to find any other lists or organizations which we could include. The final list consisted of 982 organizations.
Next, a larger team of 70+ volunteers spent seven months (05/18/2021-12/16/2021) extracting data from the websites of these organizations, looking for:
- Whether the organization satisfied our inclusion criteria: US-based, registered 501(c)(3) nonprofit organization, focused on one or more rare diseases (one that affects less than 200,000 people in the US (Orphan Drug Act, 1983)) and having an active website.
- Basic organizational info: year of founding, name & contact information of founders
- Information as to the organization’s resources: conference, research agenda, biobank, registry, natural history study, etc.
- Whether the rare disease has treatment guidelines, or whether it is known that it is caused by a genetic mutation
- Any mention of drug repurposing, and if so - which drug(s) were pursued
- Any mention of partnerships or collaborations with other organizations
As a result of this exercise, 711 organizations were confirmed as rare disease nonprofit organizations in the US with active websites as of 12/2021. This dataset has been made available open-source at [url here].
UpToDate Data on Off-label use
Separately, we onboarded a team of 10 extractors which had some medical expertise or medical educational background. We took a subset of 100 rare diseases from our ROADMAP survey data and conducted an extraction process of identifying how many of these rare diseases had off-label drugs as a part of the official treatment guidelines on UpToDate.com, a well known and trusted electronic clinical resource tool for physicians. From 2/17/2022 to 7/14/2022, the team extracted every drug mentioned on the UpToDate page regarding a specific rare disease in the list and coded the drug entry on its “context” and “status”:
Context refers to how the drug was mentioned on the UpToDate page. The options were “Context: Drug is recommended or listed as potentially helpful” and “Drug listed as does not work for this disease AND/OR no longer given AND/OR not recommended”.
Status refers to the drugs’ approval status. The options were:
- FDA approved for another disease
- FDA approved for this rare disease
- Not FDA approved for anything
The goal of this data extraction was to understand the current state of rare disease treatment. A common statistic that is utilized everywhere is that 95% of rare diseases do not have an FDA approved treatment. As far as we know, this statistic is never cited to any original research or dataset, so it is not clear how accurate or up to date it is. Furthermore, it does not tell us how many of these rare diseases have off-label (FDA approved for another disease, but not FDA approved for that rare disease) treatments available for them. This is crucial information for us to understand the state of rare disease treatment in the US and the need for drug repurposing.
Survey Execution
We designed a survey and implemented in with the Qualtrics platform, with question sections for several different stakeholder types: (1) rare disease nonprofit organization leaders, (2) rare disease patients, (3) rare disease patients’ loved one (parent, spouse, friend, sibling, etc. of a rare disease patient), (4) physicians who treat rare diseases, (5) rare disease researchers.
Any member of the leadership team of a US-based rare disease-focused nonprofit organization were able to participate in this research project. They were then invited to directly reach out to their US-based patient, loved one, physician and researcher network and invite them to take the survey. Since rare diseases predominantly affect children, we allowed participants under 18 to participate in the project through an adult loved one who provided informed consent and took the survey. Additionally, since many rare diseases cause physical and cognitive disabilities, we allowed those patients to also participate in the survey through a loved one, regardless of the patient’s age in that case. Also, we included an option for patients and loved ones to participate in Spanish, since we anticipated that a large portion of the population might not be fluent English speakers.
Each of these stakeholders had a unique insight into drug repurposing for rare diseases that we wanted to capture:
- For rare disease nonprofit organization leaders we were focusing on capturing their organizational characteristics (age, funding, staff size, etc.), level of resources (biobank, patient registry, natural history study, scientific advisory board. etc), rare disease state of research (availability of treatment guidelines, diagnostic criteria, ICD code, biomarkers, etc.), any FDA or off label drugs utilized for their rare disease of focus as well as their efficacy, availability and access, their drug repurposing experience, including challenges they’ve encountered, support they need, and who was in their collaboration network.
- For rare disease patients and their loved ones (these stakeholders received the same set of questions) we were interested in their experience with off label drugs for their rare disease, including roadblocks in access, as well as their general attitude towards and comfort level with drug repurposing.
- For physicians, we were mainly interested in the frequency and factors taken into consideration when deciding to prescribe a drug off-label, off-label drug prescription practices for the rare diseases they treat, as well as their levels of involvement in clinical trials for rare diseases.
- For researchers, we asked questions regarding their involvement in research projects to advance drug repurposing for any rare diseases, what their motivations are, what challenges they’ve encountered.